Recently, there have been increasing concerns about the impact of the increase in expensive healthcare technologies on medical insurance finances, and trial implementation of cost-effectiveness evaluations on medicines and medical devices began in fiscal 2016. From April 2019, the full-scale introduction of cost-effectiveness evaluations will finally begin in Japan. In other countries, there are already many public health technology assessment (HTA) agencies responsible for these tasks. In response to these changes, the Center for Outcomes Research and Economic Evaluation for Health (C2H) was established in Japan in April 2018 within the National Institute of Public Health. The following symposium was held for the purpose of having personnel from the HTA agencies of other preceding countries, such as NICE in the UK, explain the mechanism of health technology assessment in their respective countries and to also promote the importance of cost-effectiveness evaluations in Japan.
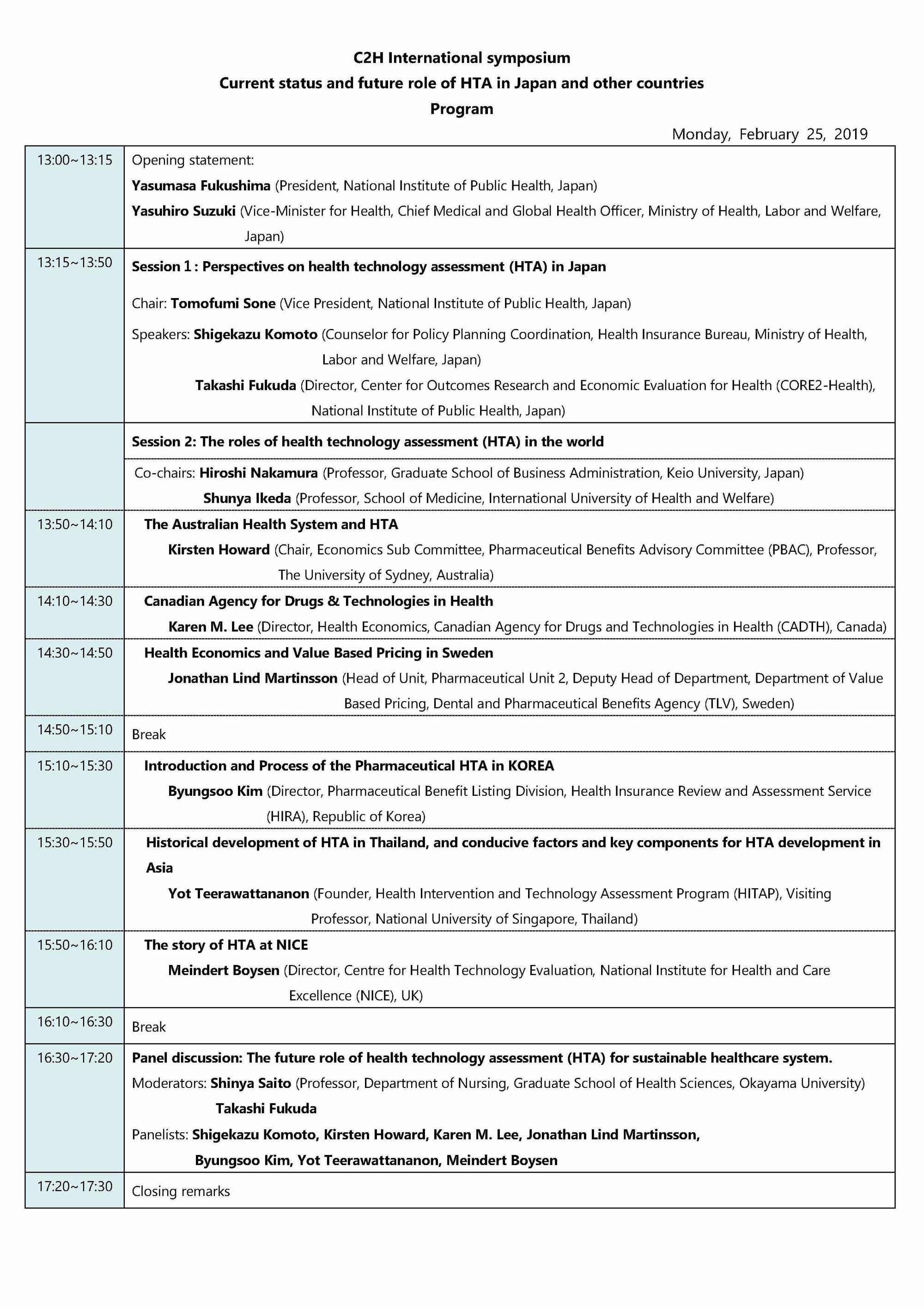
Date and Time: Monday, February 25, 2019 1 pm to 5:30 pm
Theme: Current Status and Future Role of HTA in Japan and Other Countries
Venue:The GRAND HALL Shinagawa (3rd Floor, Shinagawa Grand Central Tower, 2-16-4 Konan, Minato-ku, Tokyo)
Presentation materials
- Shigekazu Komoto, Counselor for Policy Planning Coordination, Health Insurance Bureau, Ministry of Health, Labor and Welfare, Japan, Perspectives on health technology assessment (HTA) in Japan [1.4MB]
- Kirsten Howard, Chair, Economics Sub Committee, Pharmaceutical Benefits Advisory Committee (PBAC), The Australian Health System and HTA [381KB]
- Karen Lee, Director, Health Economics, Canadian Agency for Drugs and Technologies in Health (CADTH), Canadian Agency for Drugs and Technologies in Health [352KB]
- Jonathan Lind Martinsson, Head of Unit, Pharmaceutical Unit 2, Deputy Head of Department, Department of Value Based Pricing, Dental and Pharmaceutical Benefits Agency (TLV), Health Economics and Value Based Pricing in Sweden [741KB]
- Byungsoo Kim, Director, Pharmaceutical Benefit Listing Division, Health Insurance Review and Assessment Service (HIRA), Introduction and Process of the Pharmaceutical HTA in KOREA [2.5MB]
- Yot Teerawattananon, Founder, Health Intervention and Technology Assessment Program (HITAP), Historical development of HTA in Thailand, and conducive factors and key components for HTA development in Asia [1.2MB]
- Meindert Boysen, Director, Centre for Health Technology Evaluation, National Institute for Health and Care Excellence (NICE), The story of HTA at NICE [690KB]